How Injection Molded Medicine Containers Solve Common Pharmaceutical Packaging Issues
Release time:
Sep 21,2025
Key Takeaways
- Injection molded medicine containers protect medicines from moisture and heat, ensuring their safety and effectiveness.
- Using medical-grade materials like polypropylene and high-density polyethylene helps maintain the integrity of pharmaceutical products.
- Implementing strict quality control measures reduces defects and ensures compliance with safety regulations.
Injection Molded Quality and Safety

Material Selection
You need medicine containers that guarantee drug safety and biocompatibility. Texin selects only medical-grade materials for injection molded containers. These materials include polypropylene (PP), high-density polyethylene (HDPE), and polycarbonate (PC). Each material offers unique properties that support the integrity of your pharmaceutical products.
Here is a comparison of common medical-grade materials and their applications:
You benefit from these materials because they resist moisture, chemicals, and heat. PP and HDPE provide excellent chemical resistance and low moisture uptake, making them ideal for single-use medicine containers. PC offers clarity and withstands autoclave sterilisation, which is essential for applications that require visibility and heat resistance. These properties help prevent contamination and maintain the quality of your medicines.
Quality Control Measures
You rely on consistent quality in every batch of injection molded containers. Texin implements strict quality control at every stage of the process. Incoming inspections ensure that only approved medical-grade resins enter production. Scientific injection molding techniques set precise parameters for temperature, pressure, and cooling. This approach reduces mistakes and minimises injection molding defects such as warping or incomplete filling.
Texin uses advanced moulds made from hardened steels like S136 and H13. These moulds deliver high-quality finishes and long-lasting performance. During production, real-time monitoring systems track each step. You receive containers with full traceability, as each lot carries a resin batch number and production date. Comprehensive inspections confirm that every container meets your specifications before shipment.
Tip: Consistent quality control measures help you avoid costly defects and ensure regulatory compliance.
Sterilisation Compatibility
You must sterilise medicine containers to meet pharmaceutical safety standards. Texin designs injection molded containers to withstand multiple sterilisation methods. These include ethylene oxide (EtO), gamma irradiation, and steam autoclaving. Each method has unique effects on the physical properties of plastics.
You can choose the sterilisation method that best fits your application. PP and HDPE resist EtO and gamma sterilisation, while PC handles steam autoclaving. This flexibility ensures that your injection molded containers maintain their quality and safety after sterilisation. You avoid warping and other molding defects that can compromise product integrity.
Note: Selecting the right material and sterilisation method increases efficiency and reduces the risk of defects during production.
Injection Molding Defects and Solutions
Common Defects
You may encounter several common injection molding defects when producing medicine containers. These defects can affect both the appearance and function of your packaging. Understanding these issues helps you maintain high product quality and avoid safety concerns.
You might also see voids, which are internal gaps caused by improper filling, and surface delamination, where layers of material separate. These material defects can compromise the safety and reliability of your injection molded containers. If you ignore these issues, you risk quality issues, reduced production efficiency, and potential safety concerns for end users.
Tip: Early identification of common defects helps you prevent mistakes and maintain product quality.
Prevention Strategies
You can reduce injection molding defects by following scientific approaches to process validation and tooling. Texin uses a three-phase validation method:
- Process Design: You define the best parameters for injection, such as temperature, pressure, and cooling time.
- Process Qualification: You test and confirm that the process produces consistent, high-quality results.
- Continued Process Verification: You monitor the process over time to ensure stability and efficiency.
This approach gives you confidence that your injection molded medicine containers meet strict standards. You avoid common defects like warping, sink marks, and short shots by using advanced tooling and precise control of the injection process. Texin’s use of hardened steel moulds and scientific injection molding techniques helps you achieve reliable results. Preventing warping and other molding defects ensures that your containers fit perfectly and protect medicines from contamination.
Note: Scientific validation and robust tooling are essential for reducing defects and improving production efficiency.
Ensuring Consistency
You need ongoing inspections and traceability systems to maintain high quality and safety. Regular inspections help you catch defects, inconsistencies, and compliance issues early in the production process. Advanced inspection systems can detect minute molding defects that manual checks might miss. This reduces the risk of defective products reaching the market.
Traceability plays a vital role in regulatory compliance and recall management. By labelling each batch with resin batch numbers and production dates, you can track every container throughout the supply chain. This allows you to isolate defective products quickly if needed, reducing the impact of recalls and protecting your brand. Traceability also supports root cause analysis, helping you identify and fix problems before they affect product quality.
Alert: Quality control measures and traceability systems are your best tools for preventing defects and ensuring safety in pharmaceutical packaging.
Sustainability and Cost Efficiency
Recyclable Materials
You play a vital role in reducing environmental impact when you choose injection molded medicine containers made from recyclable and biodegradable materials. Texin selects eco-friendly plastics that support sustainability goals and help address the growing problem of plastic waste. Only 2% of plastic packaging materials are recycled globally, so your decision to use recyclable containers makes a real difference.
- Sustainability covers human well-being, economic growth, and environmental health.
- Most plastic waste ends up in landfills or the environment, which highlights the urgent need for sustainable alternatives.
- Green pharmaceutical packaging is gaining popularity because it reduces waste and supports a cleaner planet.
You also benefit from materials that resist microbial growth, which helps maintain the quality of your medicines. By using injection molded containers, you support waste management solutions and meet regulatory standards for safety and efficacy.
Scalable Production
You need a production method that delivers both efficiency and cost savings. Injection molding enables you to scale up quickly and meet high market demand for pharmaceutical packaging. This process allows you to produce large quantities of containers with consistent quality and minimal defects, such as warping or incomplete filling.
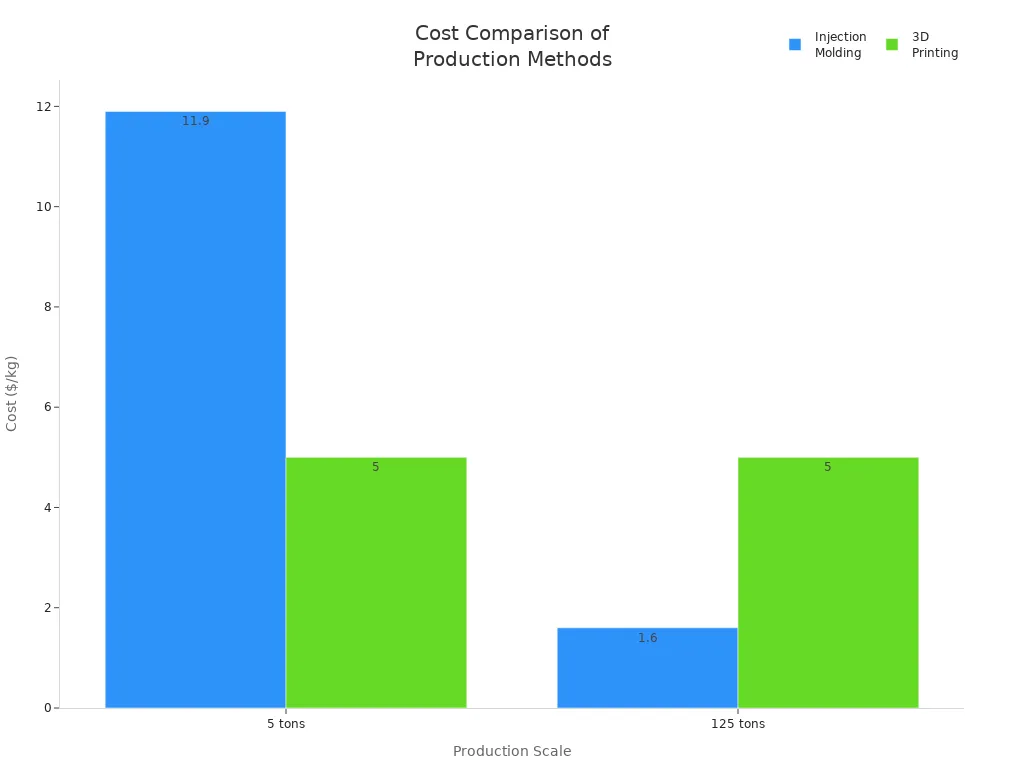
Injection molding gives you rapid production cycles, which are crucial for meeting tight deadlines. As you increase production volume, your costs drop significantly. For example, the cost per kilogram for injection molding falls from $11.9 at 5 tons to $1.6 at 125 tons—a 68% reduction. This efficiency helps you maintain high quality while keeping expenses low. You also benefit from robust quality control, which prevents defects and ensures that each batch meets your standards. By preventing warping and other molding defects, you deliver reliable packaging that protects medicines and supports patient safety.
You solve packaging challenges with Texin’s injection molded medicine containers. Precision injection molding delivers consistent results:
- Clean rooms reduce contamination and recalls.
- You protect patients and meet regulatory needs.
Choose Texin for regulatory-ready packaging.
FAQ
What materials do you recommend for medicine containers?
You should choose polypropylene (PP), high-density polyethylene (HDPE), or polycarbonate (PC). These materials offer strong chemical resistance and support pharmaceutical safety.
How do you ensure the containers remain sterile?
You can sterilise containers using ethylene oxide, gamma irradiation, or steam autoclaving. Each method keeps your packaging safe for medical use.
Can you recycle injection molded medicine containers?
Yes, you can recycle most injection molded containers. Polypropylene and high-density polyethylene are widely accepted by recycling programmes.
Related Blog
Share


